|
Case Report
Tricky papillary muscle rupture sequelae navigated by evidence-based therapies using ECMO and Impella
1 Resident Physician, Internal Medicine, Northeast Georgia Medical Center, Gainesville, GA, USA
2 FACC, Program Director, Cardiovascular Medicine Fellowship Program and Medical Director, Heart Failure Treatment and Recovery Center, Georgia Heart institute, Gainesville, GA, USA
Address correspondence to:
Christine Sykalo
DO, 743 Spring St NE, Suite 700, Gainesville, GA 30501,
USA
Message to Corresponding Author
Article ID: 100012C03CS2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Sykalo C, Herrera M, Adams A, Egolum U. Tricky papillary muscle rupture sequelae navigated by evidence-based therapies using ECMO and Impella. Edorium J Cardiol 2022;6(2):1–5.ABSTRACT
This case illustrates an atypical mitral valve prolapse presentation with superimposed stress of acute respiratory distress syndrome (ARDS) as well as the importance of extracorporeal membrane oxygenation therapy (ECMO) as a bridge to definitive surgical intervention. Switching from venovenous (VV) to venoarterial (VA) ECMO allowed bypass of the pulmonary circulation and provided cardiac support to assist in systemic circulation in the setting of severe mitral regurgitation with mitral valve prolapse. However, VA ECMO increases afterload, thus an Impella was used to offload the left ventricle and provide forward flow. Relying on evidence-based medicine for each modality despite its complexity also optimized this patient’s chance for recovery. Thereby, we demonstrate a complex case of ARDS, mitral valve prolapse secondary to myocardial infarction, and subsequent multiple arrhythmic arrests, where successful VV and VA ECMO resuscitation afforded bridge therapy to definitive surgical management. Our patient showed promising results, and we would like to encourage this strategy to bridge patients requiring surgical intervention.
Keywords: Cardiac critical care, ECMO, Impella, Valve replacement
INTRODUCTION
Papillary muscle rupture is a dreaded complication that occurs three to seven days after a myocardial infraction in about 1% of patients [1]. Frequently leading to cardiogenic shock, occurrences carry as high as 80% mortality rates in hospitalized patients [2]. Many patients who would otherwise be surgical candidates are too ill to undergo surgical intervention. Fortunately, extracorporeal membrane oxygenation (ECMO) and left ventricular-assisted devices offer patients life-sustaining therapy while awaiting optimization for definitive surgical intervention [3]. Occasionally, patients require bridging modalities to be safe for surgical intervention.
CASE REPORT
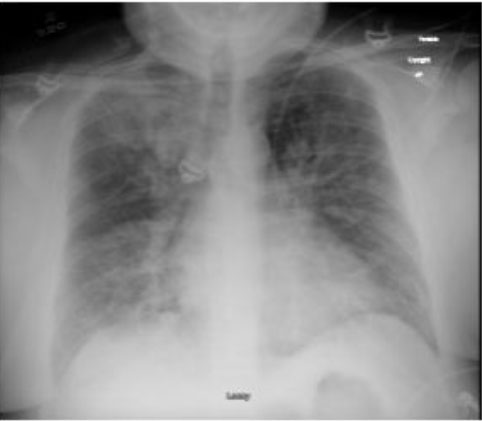
A 43-year-old male, with past medical history significant for obesity with body mass index (BMI) of 42.4, hypertension with medication non-adherence, and tobacco abuse, presented with complaint of worsening shortness of breath for two days. On admission, he was found to be hypoxic, requiring venturi mask at 60%. On presenting exam, pertinent findings were tachypnic at 25 bpm, with decreased breath sounds in the right and left lower fields, along with wheezing in the bilateral upper fields. Abnormal labs on admission included leukocytosis of 26.5 K/uL and creatinine of 1.64 mg/dL (from baseline creatinine of 1.0 mg/dL). Antibiotics, steroids, and nebulizer treatment were initiated in the emergency department with minimal improvement. Suspected pulmonary embolism was ruled out by pulmonary computed tomography angiography (CTA), but the patient was found to have troponin elevation of 0.51 that increased to 1.78 (normal assay < 0.10), with no ST elevations on electrocardiogram (EKG). This was suspected to be a Type II myocardial infarction in setting of severe illness. A chest X-ray demonstrated bilateral lung disease with patchy infiltrates suspicious for pneumonia, with possible underlying interstitial lung disease or volume overload (Figure 1). During this initial diagnostic exam, the patient experienced worsening respiratory distress, tachypnea of 40 bpm, tachycardia, and inability to speak, requiring intubation with paralysis for vent synchrony. The patient required vasopressors and was admitted to the critical care unit, initiated on the Marik protocol, and a bronchoscopy was performed.
At this time, the patient was diagnosed with an acute renal injury with no signs of acute renal abnormalities on renal ultrasound, a type II NSTEMI, and acute respiratory failure of multifactorial etiology. An echocardiogram demonstrated EF of 60–65% with grade 3 diastolic failure and mild mitral regurgitation, presumed to be a contributing factor of patient’s presentation. By the fourth day, the patient was on empiric antibiotic treatment, but his respiratory status continued to decline and he was unable to tolerate supine ventilation. A repeat chest X-ray showed acute respiratory distress syndrome (ARDS), and the patient planned to be initiated on venovenous (VV) extracorporeal membrane oxygenation (ECMO), once the equipment was available. On the fifth day, after suctioning patient and collecting respiratory cultures, patient’s oxygen saturation dropped to 74% and bag mask ventilation along with airway pressure release ventilation were unable to significantly improve patient’s oxygenation, and VV ECMO cannulation was performed via right internal jugular vein. The patient developed atrial fibrillation with rapid ventricular response, and amiodarone drip was added to his treatment, but heart rate remained in the 160 bpm. The following day, the patient received a percutaneous tracheostomy. On the eighth day, the patient developed bradycardia to asystole after a coughing episode, with spontaneous return of circulation. During this time period, the patient received multiple bronchoscopies for his ARDS that demonstrated only clear-yellow fluid. Throughout this time his creatinine continued to increase, with suspected acute tubular necrosis.
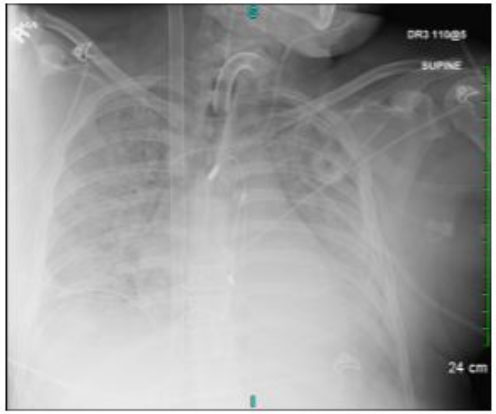
The patient was noted to have premature ventricular contractions (PVCs) on telemetry along with desaturations. Another EKG and X-ray were performed, showing evidence of new ST depressions in anterolateral leads, and persistent bilateral lung consolidations. Given the new cardiac findings, a repeat transthoracic echocardiogram was obtained, which showed severe prolapse of posterior mitral leaflet with possible ruptured posteromedial papillary muscle, mild prolapse of anterior mitral leaflet holosystolic mitral valve prolapse, and likely severe regurgitation, but was difficult to assess with certainty due to body habitus. The inferior vena cava (IVC) and right ventricle were also noted to be dilated, and transesophageal echocardiography (TEE) was done to further delineate mitral regurgitation. The TEE confirmed the previous findings of severe mitral regurgitation with a malcoaptation of mitral valve and torrential mitral regurgitation, without thrombus in left atrial appendage. Continuous renal replacement therapy (CRRT) was planned for volume overload due to the IVC dilation. While awaiting Cath Lab to assess coronary anatomy and likely proceed with mitral valve intervention in the coming days, the patient suffered ventricular fibrillation requiring cardiopulmonary resuscitation (CPR) for 10 minutes, along with multiple boluses of epinephrine and amiodarone. Once return of spontaneous circulation was achieved, the patient continued to have frequent PVCs with tachycardia and once again became hypotensive. The decision was made to perform arterial cannulation for venoarterial (VA) ECMO (Figure 2). Once this was completed, there was notable improvement in hypotension, tachyarrhythmia, and oxygen saturation.
On the 12th day, coronary angiogram was performed showing only mild two vessel disease. An Impella was placed to improve oxygenation and overall lung function, prior to salvage valve replacement. Continuous renal replacement therapy was also initiated with good volume removal. Over the next few days, the patient’s renal function improved with decrease in creatinine. On the 16th day, Impella was removed and the patient received salvage replacement of the mitral valve, during which time decannulation of the common femoral artery ECMO was performed, and changed to internal jugular two-stage ECMO. The patient developed atrial fibrillation and was treated with direct current cardioversion. By the 21st day, patient’s VA ECMO was decannulated and a vascular catheter was placed instead. The patient was taken off all pressors, had been on a total of five during the previous weeks.
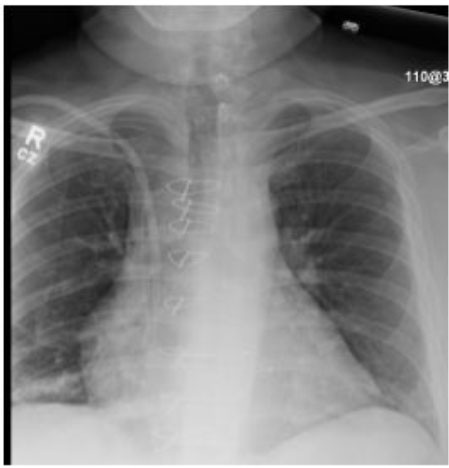
On the 24th day of hospitalization, the patient was noted to be awaken and alert. On the 25th day, the patient was able to track with his eyes and follow commands, and his oxygen requirements were slowly weaned with a T-piece trial. Over the next few days, the patient continued to improve (Figure 3). His tracheostomy collar was downsized, and he was able to sit up in a chair. His tracheostomy tube was removed, and a permacath was placed for dialysis. The patient continued to improve, passed a swallow study, and was able to tolerate a regular diet. On the 32nd day, the patient was transferred to the medical floor from the critical care unit. Subsequently, the patient was discharged to a rehabilitation facility and has been doing well since then.
DISCUSSION
Our patient presented with shortness of breath, which lead to cardiovascular arrest requiring resuscitation. Upon initial presentation, a venti-mask was presumed to be sufficient, but ECMO was required for true recovery. When ECMO was initially introduced, studies were unable to show its success until the CEASER trial came out. Now, ECMO is still controversial, but is used more frequently for respiratory failure and cardiovascular support [4].
Our patient had no known valvular disease, but spontaneously developed mitral valve regurgitation with papillary muscle rupture. This was likely secondary to his acute illness following an NSTEMI, as mechanical complications are seen following myocardial infarctions and can lead to cardiogenic shock [1]. However, papillary muscle rupture is more frequently seen with inferior wall MI’s, rather than anterolateral as in our patient. Also, this complication is seen in less than 1% of patients with MI’s and typically occurs within 3–7 days [5], while it was noted in our patient on day 11. In the setting of inferior MI’s, the posteromedial papillary muscle is more likely to rupture given the blood supply from the RCA or left circumflex arteries [5]. In anterolateral MI’s, papillary muscle rupture is rarely seen, but tends to be the anterolateral muscle [6].
We were unable to find any other case reports showing posteromedial papillary muscle rupture in an anterolateral MI. One case report did demonstrate anterolateral muscle rupture with isolated right coronary artery (RCA) lesion, suggesting that anatomical differences in blood supply should be considered, but that embolic events should also be included on the differential [7]. Whether secondary to anatomical variations in blood supply or from superimposed stress of ARDS following an MI, it is important to recognize cardiogenic dysfunction and treat it accordingly.
Recognizing cardiopulmonary dysfunction is the first step, which must be followed by an effective treatment protocol. Valve replacement is frequently required, however, bridging is often the initial step (1). Our patient required both VA ECMO and Impella support for cardiovascular and respiratory function. There have been case reports [3] showing ECMO as a bridge to surgery in patients with papillary muscle rupture, but concomitant Impella use is not a commonly utilized strategy. The decision to use both modalities stemmed from the obvious increase in afterload created by VA ECMO. Initially, VV ECMO was used since it primarily supports the lungs by removing CO2, and provides oxygen to the blood, allowing lungs to rest. Switching from VV to VA ECMO allows to bypass the pulmonary circulation and provides cardiac support to assist in systemic circulation [8],[9],[10]. This was required after recognition of the severe mitral regurgitation with papillary muscle rupture. However, VA ECMO increases afterload, thus an Impella was used to offload the left ventricle and provide forward flow.
CONCLUSION
The Impella has been used as bridge therapy to salvage mitral valve in other studies as well, but using it in concordance with the ECMO is a recent development in medicine. We would like to show that using them together is an effective strategy in cardiogenic shock. No randomized studies have been done yet to show their efficacy, but one is on its way. A group at the University of Pennsylvania under guidance of Dr. Michael Ibrahim is working on a randomized trial comparing Impella with concomitant VA ECMO use, against VA ECMO alone for cardiogenic shock (REVERSE Trial). Our patient showed promising results, and we would like to encourage this strategy to bridge patients requiring surgical intervention.
REFERENCES
1.
Burton LV, Beier K. Papillary Muscle Rupture. 2022 Jul 7. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
[Pubmed]

2.
Valle JA, Miyasaka RL, Carroll JD. Acute mitral regurgitation secondary to papillary muscle tear: Is transcatheter edge-to-edge mitral valve repair a new paradigm? Circ Cardiovasc Interv 2017;10(6): e005050. [CrossRef]
[Pubmed]

3.
Arnáiz-García ME, Dalmau-Sorlí MJ, González-Santos JM, et al. Veno-arterial extracorporeal membrane oxygenation as a bridge for enabling surgery in a patient under cardiogenic shock due to acute mitral prosthesis dysfunction. J Saudi Heart Assoc 2018;30(2):140–2. [CrossRef]
[Pubmed]

4.
Aokage T, Palmér K, Ichiba S, Takeda S. Extracorporeal membrane oxygenation for acute respiratory distress syndrome. J Intensive Care 2015;3:17. [CrossRef]
[Pubmed]

5.
Kim MY, Park CH, Lee JA, Song JH, Park SH. Papillary muscle rupture after acute myocardial infarction—The importance of transgastric view of TEE. Korean J Intern Med 2002;17(4):274–7. [CrossRef]
[Pubmed]

6.
Jayawardena S, Renteria AS, Burzyantseva O, Lokesh G, Thelusmond L. Anterolateral papillary muscle rupture caused by myocardial infarction: A case report. Cases J 2008;1(1):172. [CrossRef]
[Pubmed]

7.
Stefanovski D, Walfisch A, Kedev S, Tager S. Isolated right coronary lesion and anterolateral papillary muscle rupture – Case report and review of the literature. J Cardiothorac Surg 2012;7:75. [CrossRef]
[Pubmed]

8.
Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015;7(7):E166–76. [CrossRef]
[Pubmed]

9.
Jalil B, El-Kersh K, Frizzell J, Ahmed S. Impella percutaneous left ventricular assist device for severe acute ischaemic mitral regurgitation as a bridge to surgery. BMJ Case Rep 2017;2017:bcr2017219749. [CrossRef]
[Pubmed]

10.
Ibrahim M. Impella CP With VA ECMO for Cardiogenic Shock (REVERSE). 2018. [Available at: https://clinicaltrials.gov/ct2/show/NCT03431467]

SUPPORTING INFORMATION
Author Contributions
Christine Sykalo - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Martin Herrera - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Alex Adams - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ugochukwu Egolum - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Christine Sykalo et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.